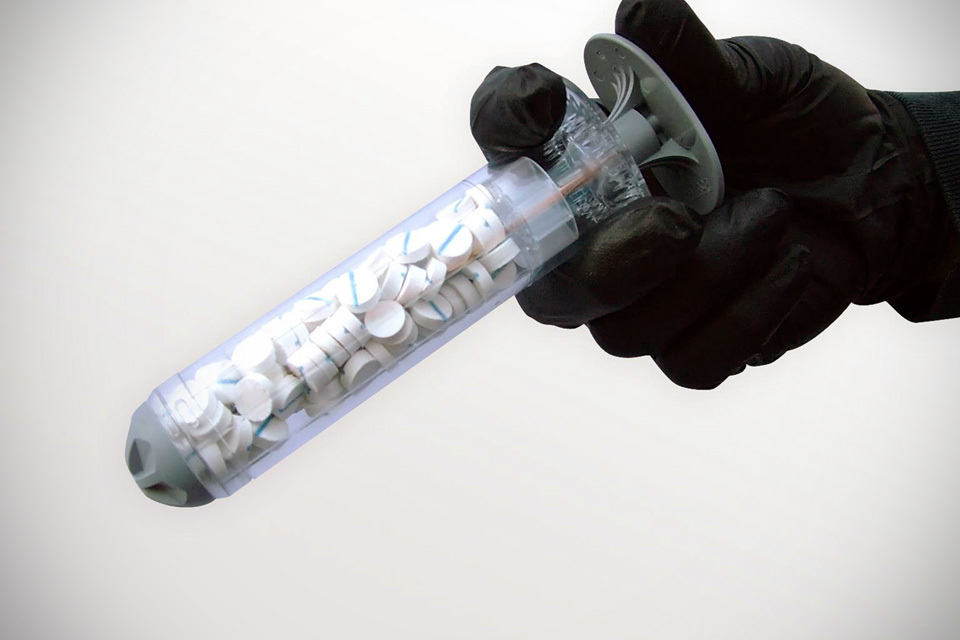
It is a good thing that most civilians won’t experience being shot by a gun. However, we can’t say the same if you are in stateside. Well, don’t get me wrong. If you have been following us long enough, you will know we are not against guns, but there are very bad sheep out there who are hell bent in tarnishing the image of gun ownership. Anyways, in what might be a life-saving milestone, U.S.’s Food and Drug Administration (or more popularly known as FDA) has recently approved a sponge-filled syringe that would help to temporary stem haemorrhages inflicted by gun shots.
This ground-breaking medical product, officially called XSTAT 30 wound dressing, was originally developed for military use, but with the recent FDA’s approval, first responders will soon be able to apply it on adults and adolescents civilian casualties. XSTAT 30 aims to stop life-threatening bleeding in areas where traditional tourniquet cannot be applied, such as the groin or armpit. Though it is not without limitations; certain areas, such parts of the chest, abdomen, pelvis or tissue above the collarbone, are no-go for this revolutionary medical device.
No less than 92 tablet-sized cellulose sponges are packed into the special syringe-style applicator you see above. Application involves introducing these tiny sponges into a wound cavity, which when upon contact with blood, will quickly expand to seal the wound, thus prevent further blood loss. XSTAT 30 serves as a temporary measure to stop bleeding for up to four hours, providing much needed time for the patient to receive proper surgical care in the nearest hospital.
According to United States Army Institute of Surgical Research, haemorrhaging is the main cause of deaths in civilian casualties and more often than not, those deaths happen before the patient reaches a hospital. In this respect, XSTAT 30 should increase the odds of survival for victims of gun shot wounds, which to us, is a welcome news. Though, nobody wish to put themselves through such agonizing pain, but it is good to know such a medical device will available soon to prevent loss of life in the event of such unfortunate incident.
But just how reliable is such a device? Well, according to William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, “when a product is developed for use in the battlefield, it is generally intended to work in a worst-case scenario where advanced care might not be immediately available.” So there you have it, a proven potential life-saving medical device for life preservation and kudos to FDA for giving it the nod of approval.
via Ubergizmo